H2CO3 Lewis Structure, Molecular Geometry, Hybridization, and MO

Estructura De Lewis Del H2co3 lios
Carbonic acid is a molecule which contains one carbon atom, three oxygen atom and two hydrogen atom. In the lewis structure of carbonic acid (H 2 CO 3 ), carbon atom is the center atom and there are two -OH groups. Also, there is one double bond between carbon and oxygen atoms. As some molecules. there are no lone pairs on carbon atom.

Nome Do ácido H2co3 AskSchool
Exercise Draw Lewis dot structures for CH 4, NH 3, HF, OF 2, F 2, O 2, N 2, Cl − and some compounds you know. Formal Charge The formal charge on any atom in a Lewis structure is a number assigned to it according to the number of valence electrons of the atom and the number of electrons around it.

lewis dot structure of h2co3 is Chemistry 16239199
Contents show Lewis Structure of Carbonic Acid (H2CO3) The formula of carbonic acid is H2CO3. It has two H atoms, one C atom, and three O atoms. To understand the molecular formula of H2CO3, we have to observe the electronic configuration of the participating atoms and how many atoms they have in the outer shell.

H2CO3 Lewis dot structure YouTube
This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
H2CO3 Lewis Structure How to Draw the Lewis Structure for Carbonic
Lewis structure of H2CO3 (Carbonic acid) contains one double bond between the Carbon atom (C) & one Oxygen atom (O) and the rest other atoms are single bonded with each other. The Carbon atom (C) is at the center and it is surrounded by one Oxygen atom (O) and two O-H bonds. All the three Oxygen atoms have 2 lone pairs.
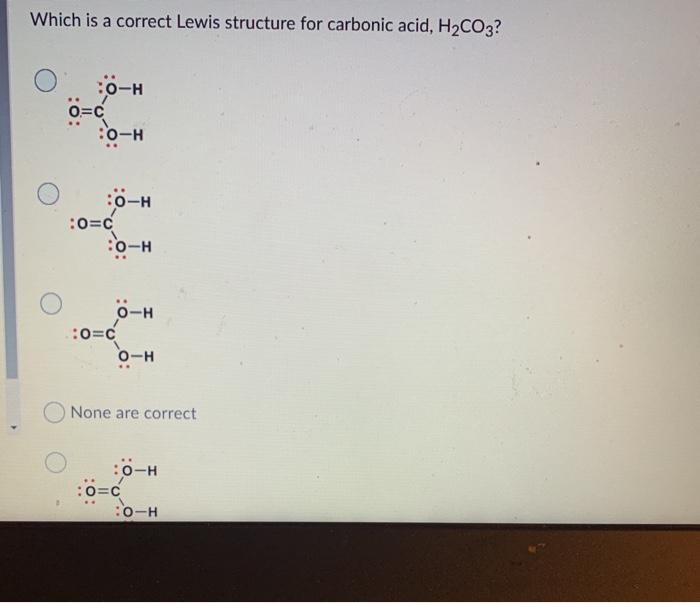
(Get Answer) Which Is A Correct Lewis Structure For Carbonic Acid
Lewis Symbols. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table.

Estructura de lewis H2CO3, enlace covalente normal polar YouTube
About this video - Lewis dot structure of H2CO3 and its hybridisation.H2CO3 also known as Carbonic Acid.Happy Reading :)
H2CO3 Carbonic acid molecule Royalty Free Vector Image
In the H 2 CO 3 Lewis structure, there is one double bond and two single bonds around the carbon atom, with three oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the left oxygen and right oxygen atom (with which the hydrogen atom is attached) also has two lone pairs. Contents Steps
[Solved] What will be the charge of the ion formed from each of these
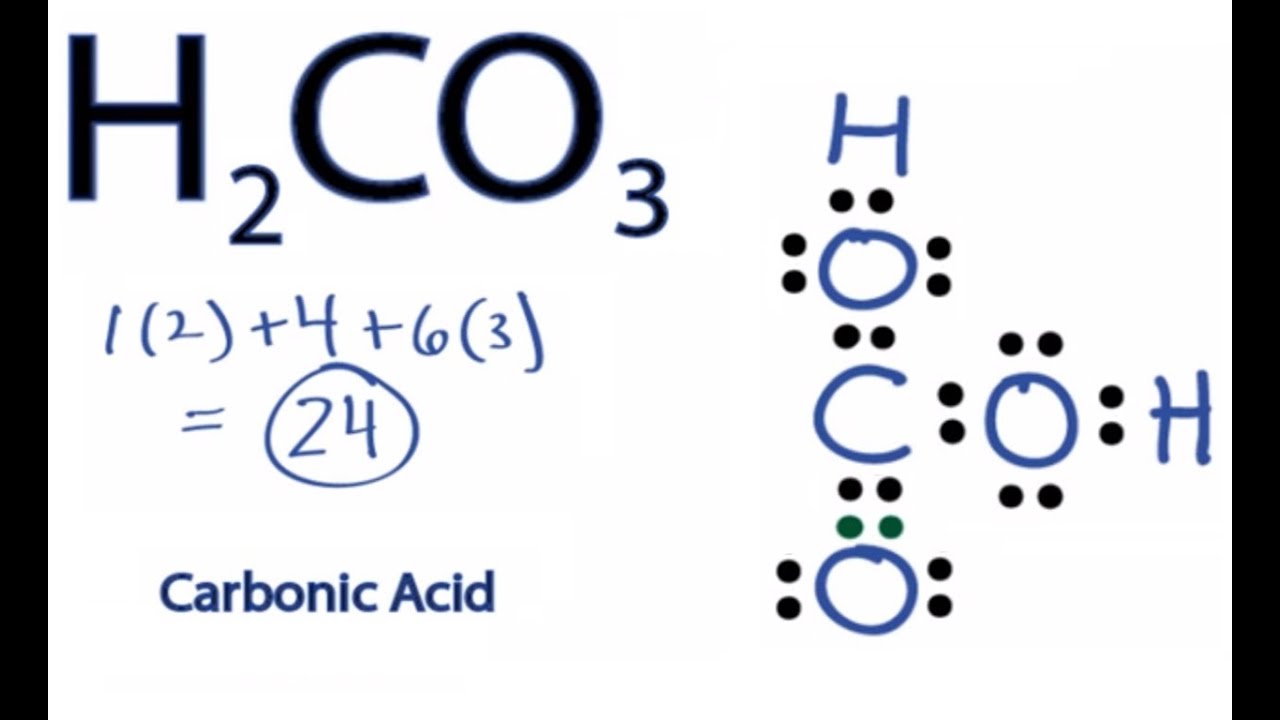
For the H 2 CO 3 Lewis structure (Carbonic Acid) make sure you put the Hydrogen atoms on the outside of the oxygen atoms. With H 2 CO 3, Carbon (C) is the least electronegative and goes in the center of the structure. There are a total of 24 valence electrons in H 2 CO 3. H2CO3 Lewis Structure: How to Draw the Lewis Structure for Carbonic Acid

[Chemistry] Lewis Structure for H2CO3 Does it matter how it looks
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the.

H2co3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
H2CO3 is one of the most known chemicals and is a chemical formula for Carbonic acid. In today's video, we help you determine its Lewis Structure by followin.

What is the Lewis structure of \ce{H2CO3}? Quizlet
A step-by-step explanation of how to draw the H2CO3 Lewis Structure (Carbonic Acid). When we have an H (or H2) in front of a polyatomic molecule (like CO3.

Lewis dot structure of carbonic acid H2CO3 Chemistry Net
Steps for drawing the Lewis dot structure of H2CO3 1. Count the total valence electrons in H2CO3 The very first step while drawing the Lewis structure of H 2 CO 3 is to find the total valence electrons present in its concerned elemental atoms.

Vetores de Ácido Carbônico Molécula Química Fórmula E Molécula Modelo
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

H2CO3 Lewis Structure, Molecular Geometry, Hybridization, and MO
http://chem-net.blogspot.com/2011/05/lewis-structures-and-octet-rule-simple.htmlhttp://chem-net.blogspot.com/2013/10/lewis-dot-structure-carbonic-acid.htmlIs.

H2co3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents (Lewis Electron Dot Structures). Let us examine the case of carbonic acid H2CO3. The Lewis structures of carbonic acid are going to be drawn using the above method